Hydrofracturing, commonly referred to as hydrofracking, is a process that is used to increase the flow of water into a well. The process can take place at the time a new well is constructed or it can be used at any time on an existing well with low or declining yield. The process involves injecting high-pressure water through the drilled well into the rock formations surrounding it. Hydrofracking may widen fractures in the bedrock to increase water to the well. In the state of Maine, hydrofracking can only be performed by a licensed water well driller.
How does it work?
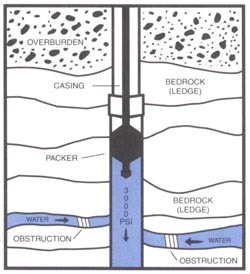
The procedure involves lowering down into the well one or two inflatable hard rubber “sleeves” or “balloons” (packers as they are more correctly called). First, all pipes, wires and the pump need to be removed from the well. The packers are then inflated to seal off a section of the well. The packers are usually set a minimum of 40 feet below the end of the casing and 60 feet below ground surface. Water is pumped at high pressure into the section of the well between the packers, or below the packer if only one is used. Most hydrofracking equipment for residential wells can provide between 500 and 3000 psi (pounds per square inch) pressure. Up to 50 gallons a minute is usually adequate as a pumping rate for adding water into the well. The water pressure within the sealed-off section of the well will rise as the surrounding rocks resist the flow of water out of the well. A sign of successful hydrofracking is a sudden drop in the pressure indicating that the surrounding rocks are accepting water. If more fissures have been opened there is often a strong back-flow of turbid water when pumping into the well is stopped.
Points for a well owner to note about the hydrofracking process:
* In order to assess the effectiveness of the hydrofracking process the contractor will usually perform a “before & after” test of the well yield.
* There is the potential for the hydrofracking process to temporarily influence water levels or turbidity in a close-by neighboring well if the two wells share some of the same fractures.
* The contractor should use high-quality water (and/or water pumped in advance from the well to be pressurized) for the hydrofracking process to avoid introducing any contaminants into the aquifer.
* After hydrofracking, the contractor will normally purge the well of fine material but there could be some cloudiness in the water for a few days.
* The use of high-pressure equipment is potentially dangerous and homeowners should stay away from the wellhead when the hydrofracking equipment is pressurized.
* The beneficial effects of hydrofracking should be permanent and usually achieve a satisfactory water yield for less cost than drilling a new well.